Yellow Card - User Guideline
Funded by the U.S. Agency for International Development (USAID), the Medicines, Technologies, and Pharmaceutical Services (MTaPS) Program, implemented by Management Sciences for Health with a consortium of partners, provides pharmaceutical system strengthening assistance for sustained of 6 improvements in health system performance and to advance USAID’s goals of preventing child and maternal deaths, controlling the HIV/AIDS epidemic, and combating infectious disease threats, as well as expanding essential health coverage.
The goal of the global, five-year (2018–2023) program is to enable low- and middle-income countries to strengthen their pharmaceutical systems to ensure sustainable access to and appropriate use of safe, effective, quality-assured, and affordable essential medicines and medicine-related pharmaceutical services. To achieve this goal, the MTAPS program has the following objectives:
- Strengthening pharmaceutical sector governance
- Increase institutional and human resource capacity for pharmaceutical management and services, including regulation of medical products
- Improve availability and use of pharmaceutical information for decision making and advance global learning agenda
- Optimize pharmaceutical sector financing, including resource allocation and Use
- Enhance pharmaceutical services, including product availability and patient centered care to achieve desired health outcomes
The USAID Mission in Bangladesh has provided funding to the MTaPS to continue the work that begun under the predecessor program, Systems for Improved Access to Pharmaceuticals and Services (SIAPS) to strengthen the Ministry of Health and Family Welfare (MOHFW) and its key directorates, i.e., Directorate General of Health Services (DGHS), Directorate General of Family Planning (DGFP), and Directorate General of Drug Administration (DGDA). MTaPS has been supporting DGDA in the following regulatory functions: Registration and Marketing Authorization, Licensing Establishment, Regulatory Inspection, and Pharmacovigilance (PV).
User’s of this document:
- HealthCare facilities
- Public Health professionals
- Marketing Authorization Holder
- Consumers
About PViMS
PViMS, or the PharmacoVigilance Monitoring System, is a web-based application used to monitor the safety of medicines. The application was developed by the USAID-funded Systems for Improved Access to Pharmaceuticals and Services [SIAPS] program (2011-2018) and is implemented by the USAID-funded Medicines, Technologies, and Pharmaceutical Services [MTaPS] program (2018-2023), both led by Management Sciences for Health (MSH), a global health nonprofit. PViMS is maintained by MSH.
JBRSOFT, a leading software development company in Bangladesh, has been working closely with the Directorate General of Drug Administration and the MSH team to customise the PViMS for the country context, build the Microservice, and provide technical support to users.
PViMS Modules
1. User Data Entry through YellowCard
Description: This module allows users to report adverse drug reactions (ADRs) and other medicine-related safety issues by filling out a YellowCard form.
Features:- Easy-to-use interface for data entry.
- Comprehensive fields to capture all necessary information regarding the ADR.
User Panel
Description: A dedicated panel for users to track their submitted case reports and manage their interactions with the system.
Features:- View status of submitted reports.
- Download copies of the YellowCard with their submitted data.
- Update or add additional information to existing reports.
Admin Panel
Description: A robust management tool for administrators to oversee and manage the entire system.
Features:- Dashboard: Provides an overview of system activity, including the number of reports submitted, processed, and pending.
- Case Management: Allows administrators to review, investigate, and take action on submitted cases.
- Reporting: Generates detailed reports on pharmacovigilance data for analysis and regulatory purposes.
Interoperability with WHO UMC Center
Description: This module ensures that PViMS can communicate and share data with the World Health Organization’s Uppsala Monitoring Centre (WHO UMC), which is the global pharmacovigilance database.
Features:- Automated data exchange protocols.
- Compliance with international standards for pharmacovigilance reporting.
Live Support Panel
Description: Provides real-time assistance to users facing issues or needing help with the system. Features:
- Live chat support.
- Access to a helpdesk for troubleshooting and inquiries.
System Configuration
Description: Allows administrators to configure and customize various aspects of the system to suit specific needs and requirements.
Features:- User role management.
- Customizable reporting forms and fields.
- System settings and preferences.
User Guideline
Description: Provides comprehensive documentation and guidelines to help users understand and effectively utilize the system.
Features:- Step-by-step instructions for using each module.
- FAQs and troubleshooting tips.
- Best practices for pharmacovigilance reporting.
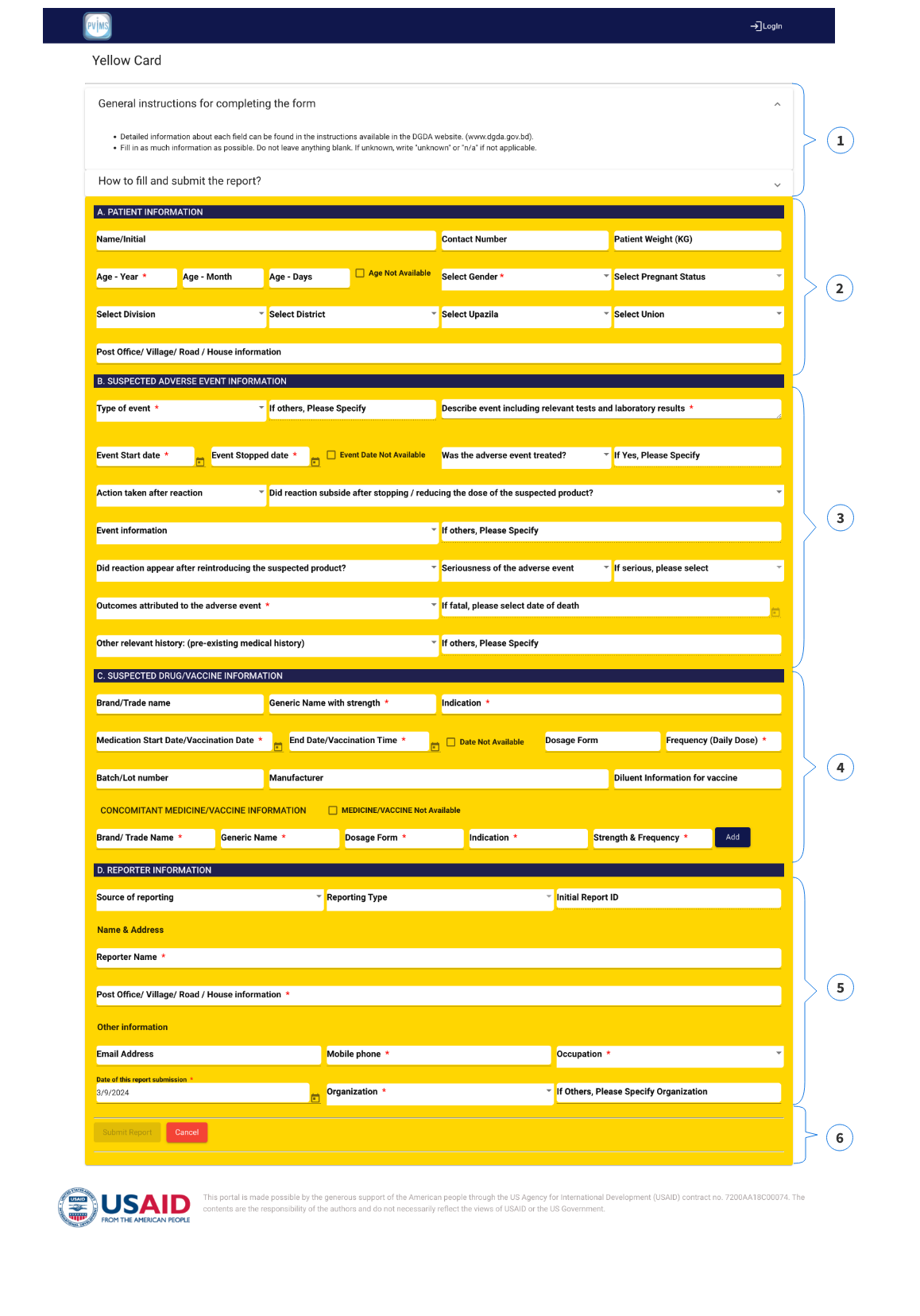
1. USER INSTRUCTIONS: You can find the user instructions in this section.
2. PATIENT INFORMATION You need to fill-out the following information:
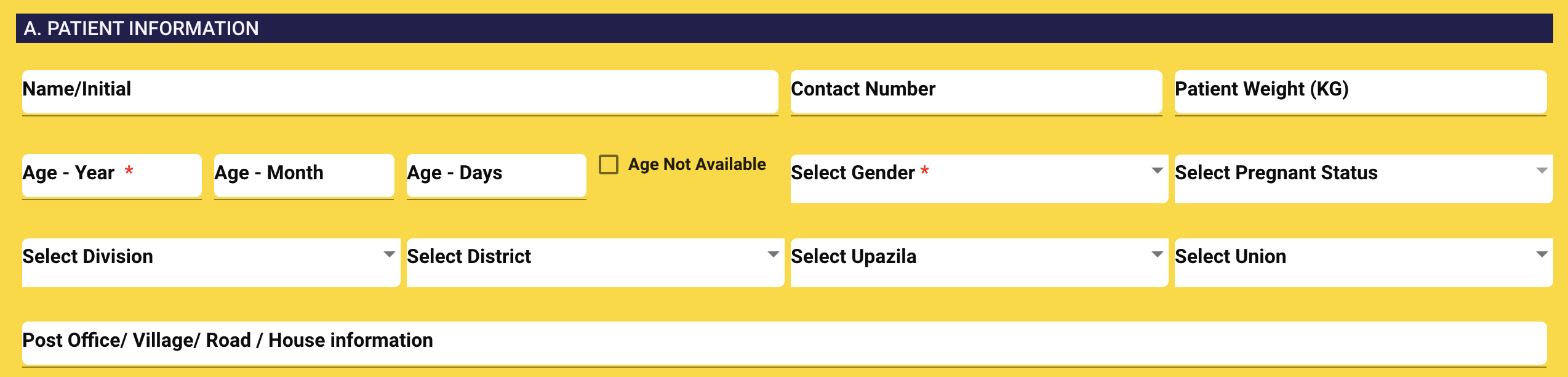
- Patient name: Enter patient fullname. It is not mandatory.
- Contact number: Enter phone number. It is not mandatory.
- Age*: Enter patient age as Year, Month and Day.
- Weight*: Enter weight in KG. It is mandatory.
- Gender*: Select gender. If you select gender as Female then another dropdown will appear with status. Please select pregnancy status.
- Division: Select division.
- District: List of districts will be appeared under this dropdown and select district.
- Thana: List of thana will be appeared under this dropdownand Select Thana.
- Street address: Enter house/ road/ village/ post office.
Notes: * refers to mandatory fields
3. SUSPECTED ADVERSE EVENT INFORMATION
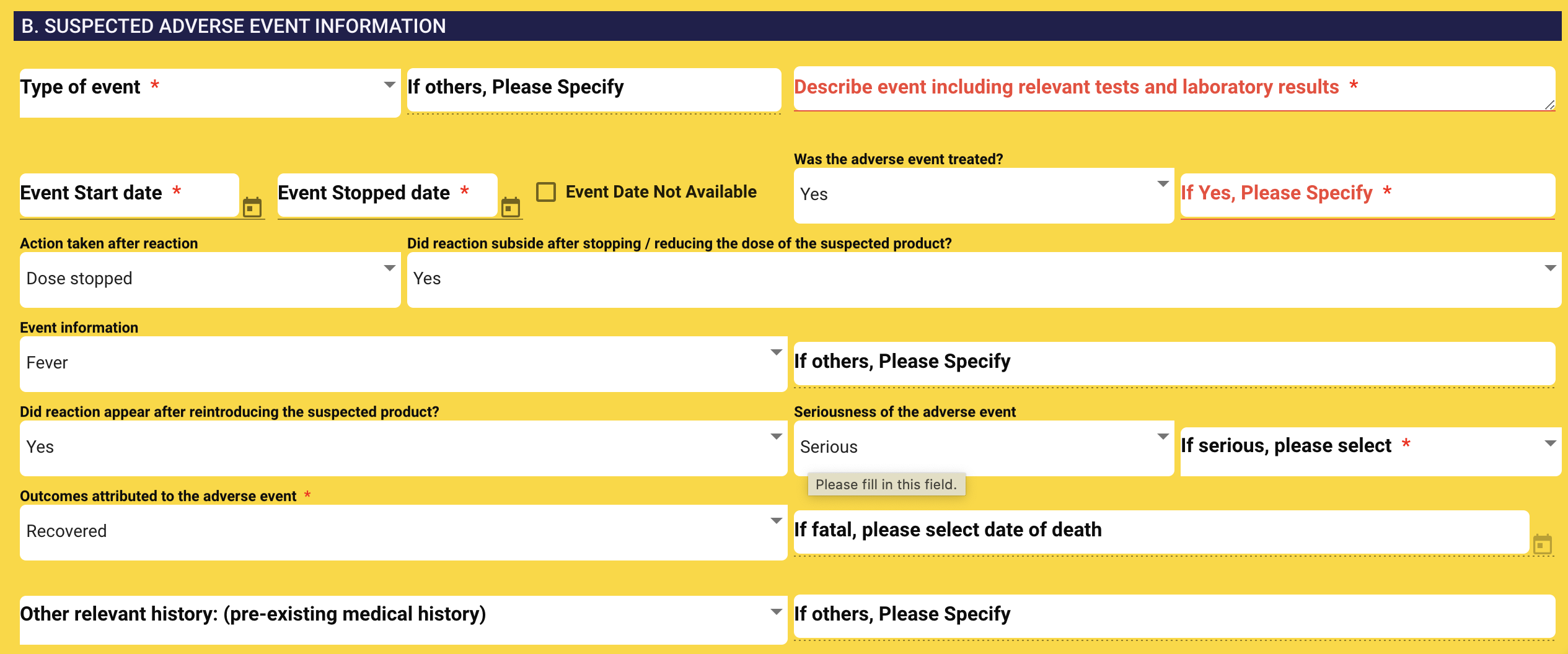
- Type of events: Select type of events (Multiple selection is allowed)
- Adverse drug reaction
- Adverse event following immunization
- Medication error
- Vaccination error
- Product quality problem
- Others
-
If other, please specify: If you select Others, a new mandatory text field named “If other, please specify” will be visible.
-
Describe event including relevant tests and laboratory results: You can provide the relevant information here.
-
Event start date: Select event start date
-
Event stop date: Select event stop date
-
Was the adverse event treated? Select event stop date
-
If yes, please specify: If you select Yes, please enter detail.
-
Action taken after reaction: Please select one action from the dropdown list.
- Dosed stopped
- Dose reduced
- No action taken
- Not available
-
Did reaction subside after stopping / reducing the dose of the suspected product?: Select Yes/No
- Please Specify: If you select Others, a new mandatory text field named “If other, please specify” will be visible.
-
Event Information:
- Fever
- Muscle pain
- Headache
- Nausea
- Vomiting
- Coughing
- Breathing difficulty
- Back pain
- Joint pain
- Abscess
- Insomnia
- High BP
- Low BP
- Increased heart rate
- Heart failure
- Myocardial Infarction
- Anaphylaxis
- Unconscious
- Others
-
Seriousness: Select Seriousness of the adverse event.
-
If serious, please specify: Multiple selection.
- Not Serious
- Hospitalization or prolongation of hospitalization
- Disability or permanent damage
- Congenital anomaly / birth defect
- Life threatening
- Death
- Other Medically important
-
Did reaction appear after reintroducing the suspected product?:
-
Outcomes attributed to the adverse event: Select outcome.
-
If fatal death, please specify
-
4. SUSPECTED DRUG/VACCINE INFORMATION
Brand of trade name: Example: Napa
-
Generic name with strength: Example: Paracetamol 500 mg
-
Indication: Example: Fever, Mild to moderate pain
-
Medication/ Vaccination start date: Select date from the calendar
-
Medication end date/ vaccination time: Select date from the calendar
-
Dosage form: Example: Child: 2-4 months 60 mg as a single dose. May give a 2nd dose after 4-6 hours if needed. Max: 4 doses daily
-
Frequency (Daily dose): TDS
-
Batch/ Lot number: Enter batch or lot number
-
Manufacturer: Enter Manufacturer information
-
Diluent Information for vaccine: Enter Diluent Information for vaccine
- Brand/Trade name:
- Generic name
- Indication
- Dosage form
- Strength and frequency
-
Source of reporting: Select reporting source
-
Reporting type: Enter reporting type.
-
Initial report ID: Enter previously submitted case ID.
-
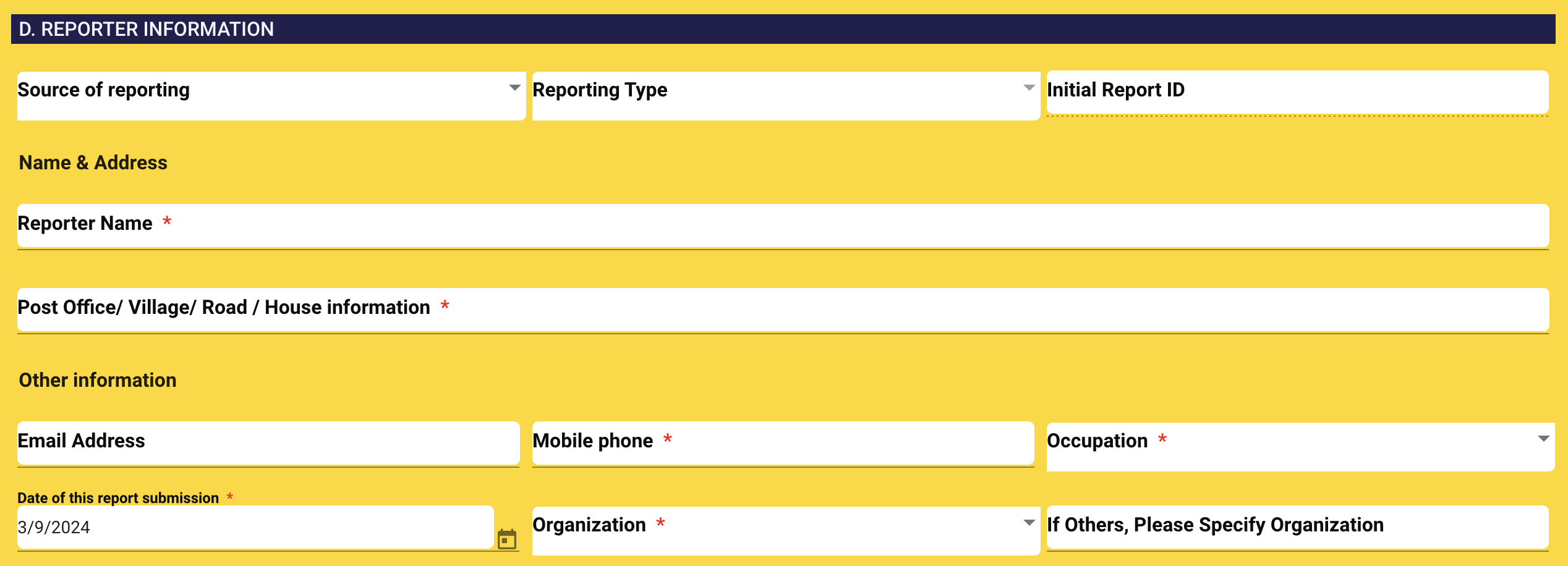
Reporter name: Enter reporter name.
-
Reporter address: Enter reporter address.
-
Reporter Email: Enter reporter email address.
-
Reporter Phone: Enter reporter phone number.
-
Reporter Occupation: Enter reporter occupation.
-
Report submission date: Select submission date.
-
Organization/ Company: Enter facility/ company or organizaiton information. It will populate following the source of reporting.
-
If other: If you don't find your organization, you can select "Other" option and specify your organization/ company name.
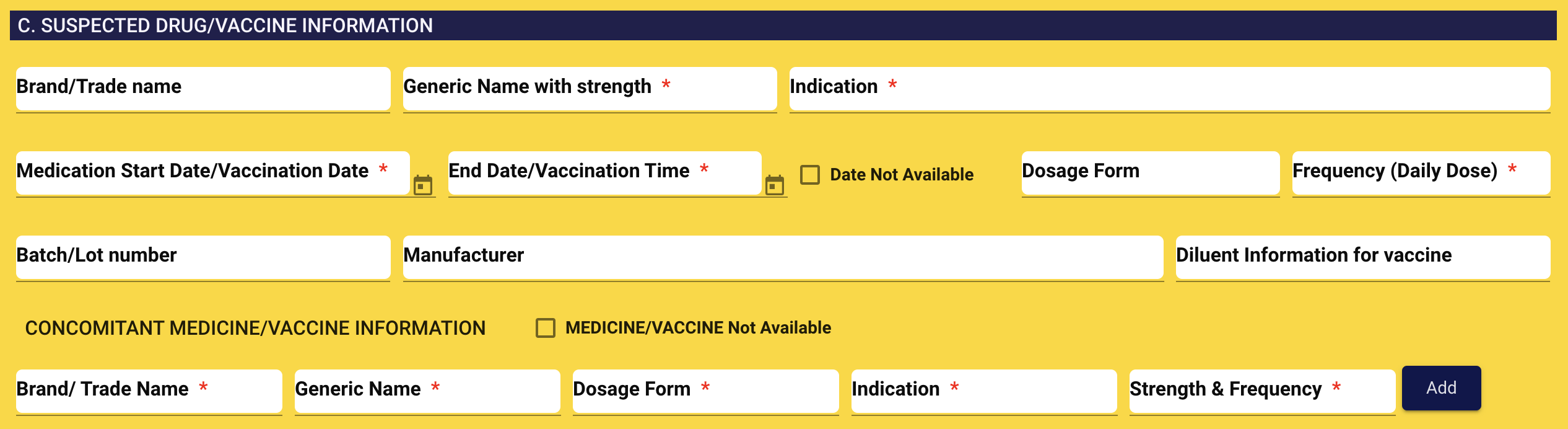
CONCOMITANT MEDICINE/VACCINE INFORMATION

5. REPORTER INFORMATION
6. REPORT SUBMISSION

Finally, click on the Sabmit Report button and submit it. An alert before Submit. Alert text: The information provided here are true to the best of my knowledge.
Once it is successfully submitted the following unique registration number will be generated:
YELLOW-CARD GENERATE AND DOWNLOAD PROCESS
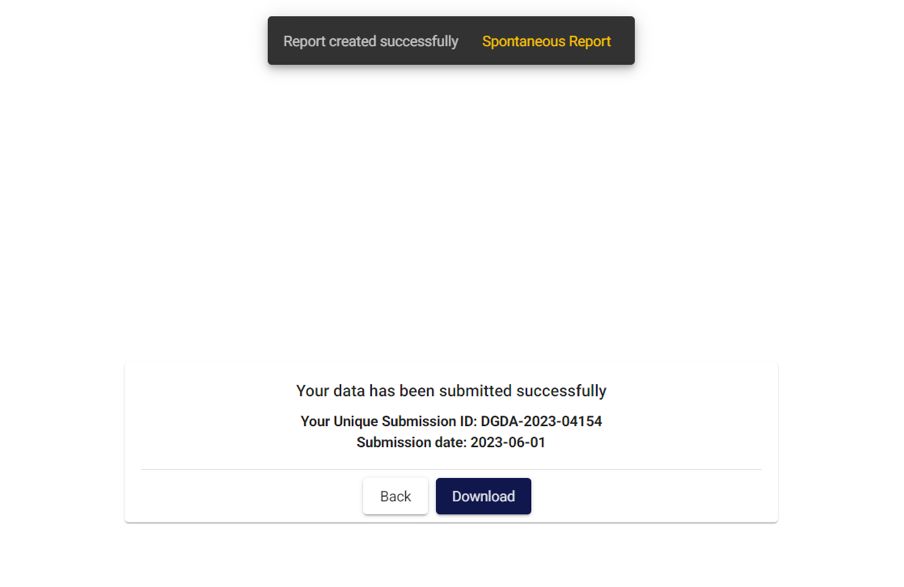
Once it is successfully submitted the yellow card from the front end then it is stored in PViMS back end. You can easily generate and download the yellow card from the Spontaneous report any time.


